Weight Loss Drug Pending Fda Approval
Weight Loss Drug Pending Fda Approval. In fact, the FDA removed dietary products with the stimulant ephedra from the U. The FDA does investigate OTC supplements if they appear to be causing harm.

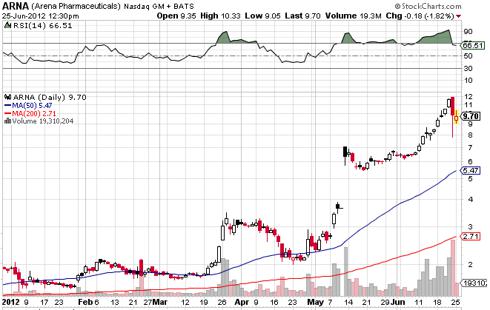
Competitive Landscape: Weight-Loss Options Weight-loss options ranged from prescription drugs to over-the-counter (OTC) remedies to various diet and The FDA would only approve Metabical as a prescription medication (vs.
Follow real-time breaking news headlines on Weight loss drugs at FETCH.news.
Physician Assistant Gerald Brown from the. It was available as a tablet (Belviq) and an extended-release tablet. Weight-loss supplements don't need FDA approval.







Post a Comment for "Weight Loss Drug Pending Fda Approval"